Fuji Capsule establishes advanced quality management systems based on strict management standards.
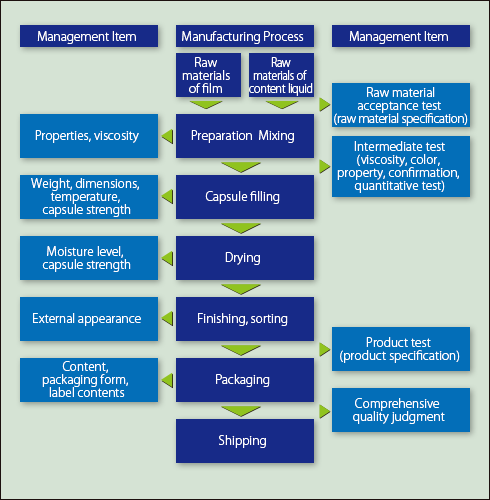
At Fuji Capsule, the quality of products is assured with inspections at each manufacturing process, raw material acceptance test, and thorough testing and inspection of intermediate and final products by skilled staff using contemporary equipment. In addition to this product testing and inspection, the quality of products is comprehensively judged based on manufacturing records etc. before shipping. Fuji Capsule adopts strict and advanced quality management systems not only for drugs, but also for quasi-drugs, cosmetics, health foods and other foods in accordance with the relevant regulations.
Product test item
| Properties | (Color, form) |
|---|---|
| Dimensions | *1 (Length, width, diameter, sphericity) |
| Weight | *2 (Gross weight, film weight, content weight) |
| Quantitative test | HPLC*3, GC*4, UV*5 |
| Physicochemical tests specific to soft capsules | Film moisture level measurement, capsule strength assessment (hardness*9, *10, *11), disintegration test*12 |
| General physicochemical tests | Moisture level, IR*6, atomic absorption, optical rotation, microscopy*7, fat and oil test (acid value, peroxide value, iodine value, hydroxyl value), TLC, pH, TOC, General Tests of Japanese Pharmacopoeia |
| Microbial test*8 | Food Sanitation Act, Japanese Pharmacopoeia |
- (Abbreviations)
- HPLC*3 = High-performance liquid chromatography
- GC*4 = Gas chromatography
- UV *5 = Ultraviolet-visible spectroscopy
- IR *6 = Infrared absorption spectroscopy
- TLC = Thin-layer chromatography
- TOC = Total organic carbon measurement method















